pentyne electron dot structure|9.5: Lewis Electron : Baguio A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom . Eye of the Kraken Slot Machine - Demo Play, Review, Payout, Free Spins & Bonuses. All you need to know about this game
pentyne electron dot structure,compound Summary. 1-Pentyne. PubChem CID. 12309. Structure. Chemical Safety. Laboratory Chemical Safety Summary (LCSS) Datasheet. Molecular Formula. C5H8. Synonyms. 1-PENTYNE. pent-1-yne. 627-19 .

The 3D chemical structure image of 1-PENTYNE is based on the ball-and-stick model which displays both the three-dimensional position of the atoms and the bonds between .
Explore the versatile organic compound, Pentyne – its structure, physical & chemical properties, applications, and safety measures. Introduction to Pentyne. Pentyne is an . A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom .Advertisement. Structure, properties, spectra, suppliers and links for: Pent-1-yne, 1-Pentyne, 627-19-0. Lewis Electron-Dot Structures. In a previous chapter, you learned that the valence electrons of an atom can be shown in a simple way with an electron dot .
A Lewis electron-dot symbol (or electron-dot symbol or a Lewis symbol) is a representation of the valence electrons of an atom that uses dots around the symbol of .Electron Dot Structures– also known as . Lewis dot structures– represent the valence electrons of atoms and show how they bond. Valence electrons are the outermost .
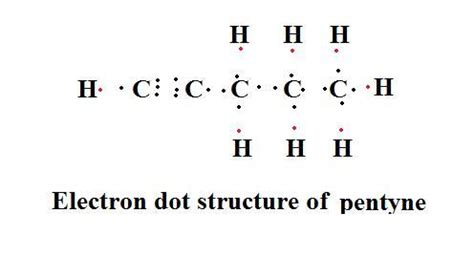
Electron dot structure of butanone,hexanal,pentanol,3 bromo pentane,pentyne, butene, - 12827121. akriti29 akriti29 05.10.2019 Chemistry Secondary School answered Electron dot structure of .
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above .
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence . A video explanation of how to draw the Lewis Dot Structure for Pentane, along with information about the compound including Formal Charges, Polarity, Hybrid .Unlike 1-pentyne, 2-pentyne is an internal alkyne, meaning the carbon-carbon triple bond is not at the end of the carbon chain. 3-Methyl-1-butyne: This variant of pentyne has a branched structure. The triple bond is located at the end of the carbon chain, similar to 1-pentyne, but there is an additional methyl (CH 3 ) group branching off the .

A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The 1-PENTYNE molecule contains a total of 12 bond (s). There are 4 non-H bond (s), 1 multiple bond (s), 1 rotatable bond (s), and 1 triple bond (s). Images of the chemical structure of 1-PENTYNE are given below:Molecular Formula: Ethane has 2 carbon atoms with 6 hydrogen atoms attached to it with a single bond. It is represented as C 2 H 6. 2. Electron Dot Formula: To draw the Lewis structure, we must keep in mind the number of valance electrons for ethane and that all the given bonds are covalent. O n the basis of the outermost shell electron .9.5: Lewis Electron Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.
Write the molecular formula of ethene and draw its electron dot structure. View Solution. Q4. Write the: (i) molecular formula (ii) electron dot formula and (iii) the structural formula of methane and ethane. [3 MARKS] View Solution. Q5. Write the equation for the following laboratory preparations:A Lewis dot structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what .Electron dot structure: “The diagram that describes the chemical bonding between atoms in a molecule is called Electron dot structure.” It is also called Lewis dot structure. Formula and electron dot structure of Cyclopentane: The formula of Cyclopentane is C 5 H 10. The electron dot structure and the structural formula can be given as
Hint: The electron dot structure which is also called as the Lewis dot structure is the representation of valence electrons of the atom around its elemental symbol in the molecule.Electron dot structure mainly shows how an atom in the molecule makes a bond by sharing the electrons. Complete answer: From your chemistry .
Lewis structure of a water molecule. Lewis structures – also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any .2-Pentyne | C5H8 | CID 12310 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. . modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise . A step-by-step explanation of how to draw the C2H5Br Lewis Dot Structure (Bromoethane).For the C2H5Br structure use the periodic table to find the total numb.
pentyne electron dot structure 9.5: Lewis ElectronPent-1-yne. Molecular Formula CH. Average mass 68.117 Da. Monoisotopic mass 68.062599 Da. ChemSpider ID 11806.
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is .
pentyne electron dot structure|9.5: Lewis Electron
PH0 · electron dot structure of pentyne
PH1 · Pentyne
PH2 · Pent
PH3 · 9.5: Lewis Electron
PH4 · 9.2: Lewis Electron Dot Diagrams
PH5 · 9
PH6 · 2.9: Electron
PH7 · 1